Surfactants (surface active agents) are important compounds for numerous oilfield applications such as fracturing, conformance control, drilling, demulsification, and enhanced oil recovery in unconventional and conventional reservoirs. The main purpose of surfactant addition is to get a favorable fluid-fluid and rock-fluid properties. For example, polymeric surfactants are used as demulsifiers to separate water from the produced oil by modifying the water/oil interface. Surfactants are also used as shale swelling inhibitors in drilling fluid formulations. In enhanced oil recovery (EOR) operations, surfactants are added to lower the water/crude oil interfacial tension (IFT) and/or to change the wettability of the rock from oil-wet to mixed- or water-wet
Most of the local reservoirs are high-temperature, high-salinity carbonate with a mixed salinity environment. The temperature of the local reservoirs is more than 100 °C and salinity varies from 120,000 ppm for “low-salinity” connate water to 220,000 ppm for “high-salinity” connate water. The injected seawater has a salinity of about 57,000 ppm. The majority of the available commercial surfactants undergo precipitation primarily because of the high salinity of the brines in most carbonate reservoirs. The harsh environment of a reservoir causes surfactant decomposition and reduces their ability in modifying the fluid-fluid or rock-fluid interface. Surfactant adsorption on reservoir rocks is another big challenge that requires a broad range of assessment studies before the application of a given surfactant in a specific reservoir. For instance, negatively charged anionic surfactants are avoided in carbonate reservoirs whereas positively charged cationic surfactants are avoided in sandstone because of adsorption challenges.
The oilfield chemistry (OFC) group at CPG is working on the molecular design of surfactants based on advanced structure-property relationships to overcome these challenges. We have mainly focused on zwitterionic surfactants and cationic gemini surfactants. Zwitterionic surfactants belong to the class of surfactants that are composed of both positively and negatively charged sites that are overall neutral. Gemini surfactants constitute a class of compounds that contain two or more lipophilic tails and hydrophilic head groups chemically bonded through a spacer. This class of compounds reveal excellent physicochemical properties such as high interfacial/surface activities. Until today, we have synthesized more than fifty cationic and zwitterionic surfactants with different head and tail groups. By tuning the chemical structure, we have accomplished a range of thermally stable and soluble gemini cationic and zwitterionic surfactants. These surfactants are mainly for EOR and equally suitable to other oilfield applications under the harsh conditions that are usually encountered in a subsurface reservoir. The molecular structures of some representative surfactants are given in Figure 1 and Figure 2. After synthesis, surfactants are characterized by NMR (Figure 3), FTIR (Figure 4), and MALDI-ToF-MS to determine the structure, purity, and molecular mass. These surfactants have shown excellent solubility at reservoir conditions (Figure 5), thermal stability, surface properties (Figure 6), and foaming properties (Figure 7). A coreflooding experiment conducted on Indiana limestone core showed a 20% incremental recovery using the locally developed surfactant (Figure 8). The developed gemini surfactants can also reduce clay swelling in different types of rocks (Figure 9)
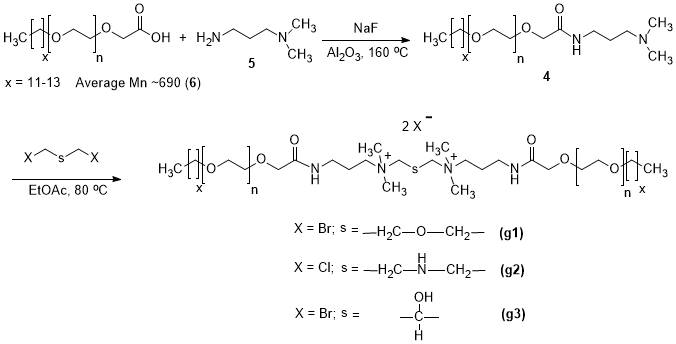
Figure 1: Typical Structures of few gemini surfactants
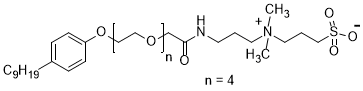
Figure 2: typical structure of zwitterionic surfactant
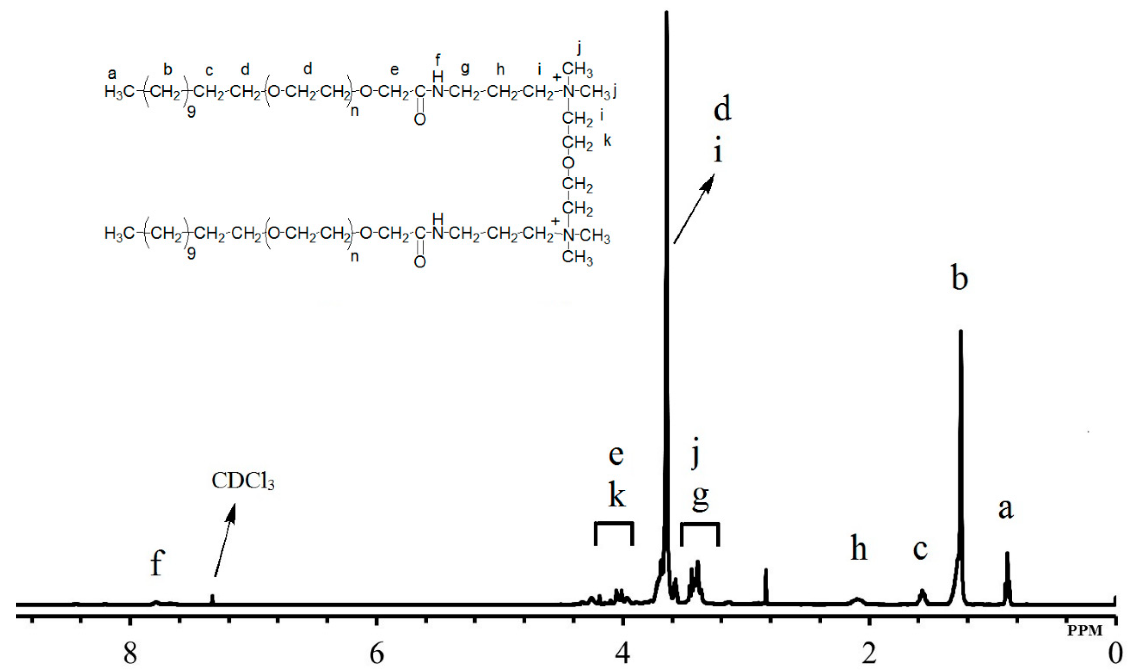
Figure 3: 1H NMR of synthesized cationic gemini surfactant
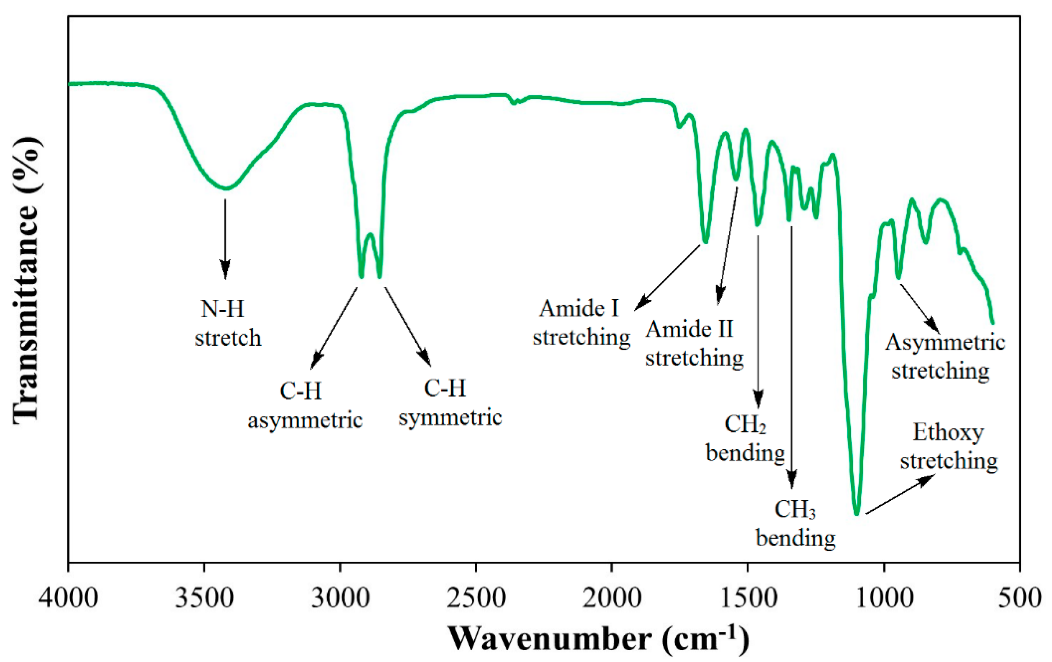
Figure 4: FTIR of synthesized cationic gemini surfactant
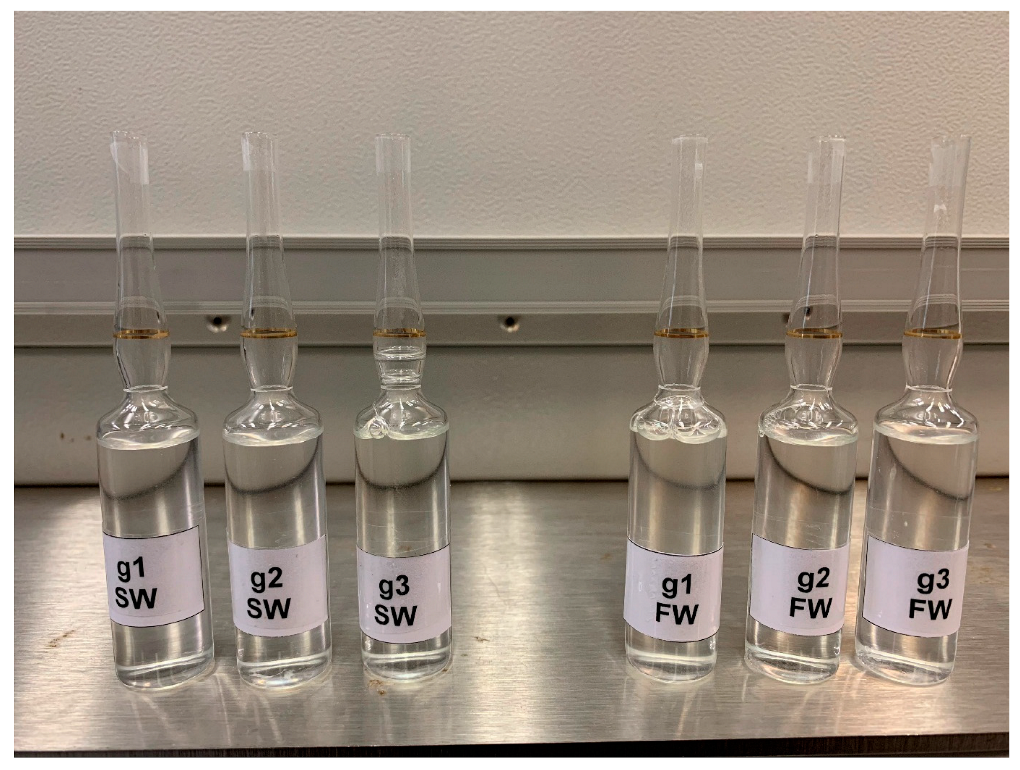
Figure 5: Solubility of the surfactants in SW and FW
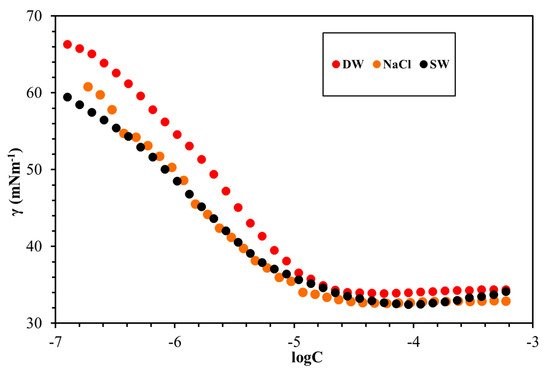
Figure 6: The surface properties of the synthesized cationic gemini surfactants
Figure 7: Foaming properties of synthesized surfactants
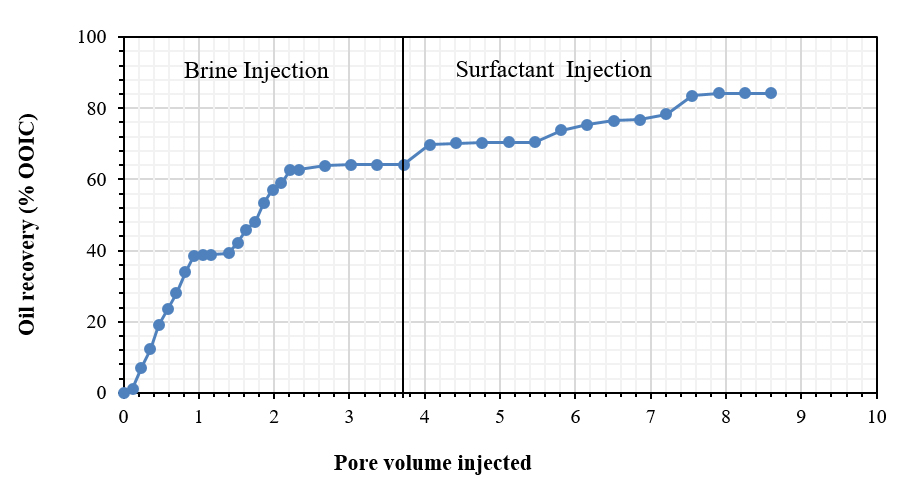
Figure 8: Oil recovery using cationic gemini surfactants
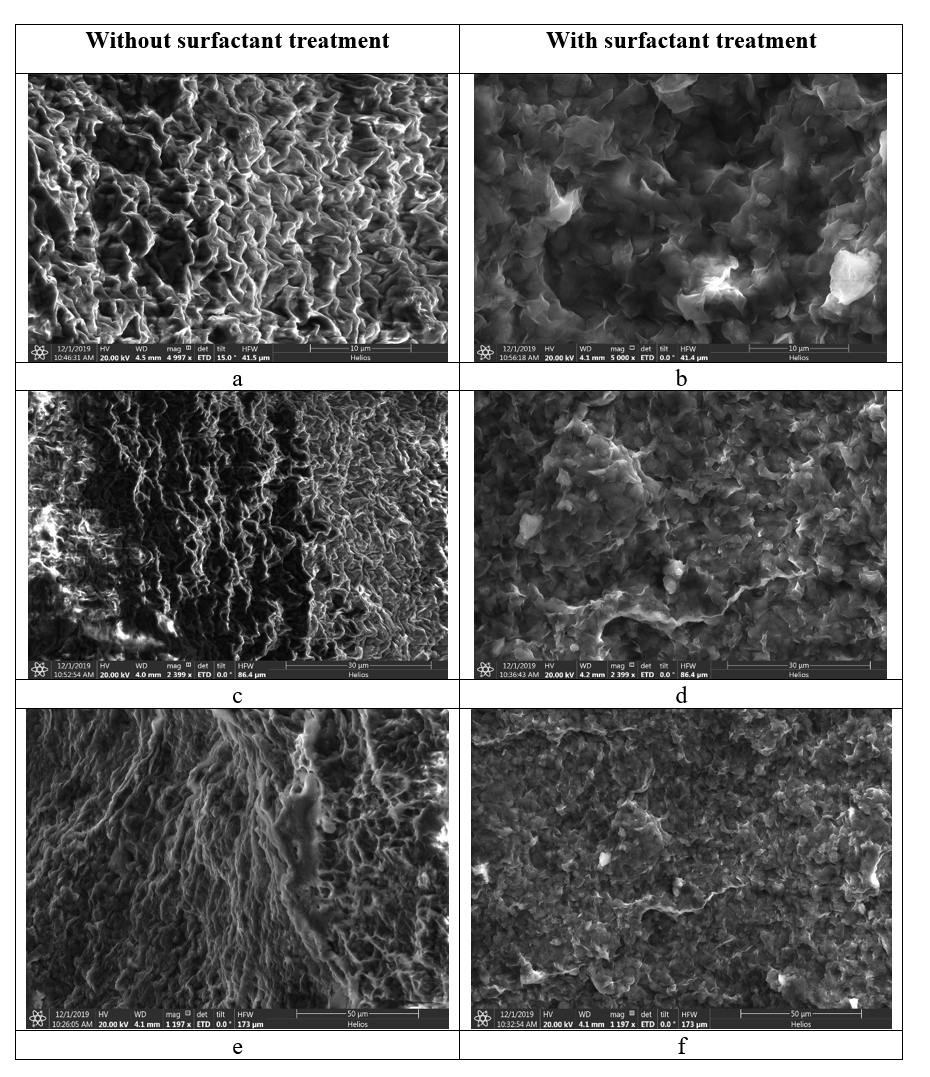
Figure 9: SEM images of a shale surface with and without surfactant treatment
The OFC research group at CPG includes Dr. Theis Solling, Dr. Syed Shakil Hussain, Dr. Kion Norrman, Dr. Muhammad Shahzad Kamal, Dr. Ahmad Adewunmi, Mr. Lionel Talley, Mr. Ahmad Mahboob, and Mr. Ali Binabdi.
References:
- Hussain, S. M.S; Kamal, M. S.; Murtaza, M. Effect of Aromatic Spacer Groups and Counterions on Aqueous Micellar and Thermal Properties of the Synthesized Quaternary Ammonium Gemini Surfactants. J. Mol. Liq. 2019, 296. https://doi.org/10.1016/j.molliq.2019.111837.
- Kamal, M. S.; Hussain, S. M. S.; Fogang, L. T. Role of Ionic Headgroups on the Thermal, Rheological, and Foaming Properties of Novel Betaine-Based Polyoxyethylene Zwitterionic Surfactants for Enhanced Oil Recovery. Processes 2019, 7 (12), 908. https://doi.org/10.3390/pr7120908.
- Hussain, S. M. S.; Kamal, M. S.; Solling, T.; Murtaza, M.; Fogang, L. T. Surface and Thermal Properties of Synthesized Cationic Poly(Ethylene Oxide) Gemini Surfactants: The Role of the Spacer. RSC Adv. 2019, 9 (52), 30154–30163. https://doi.org/10.1039/c9ra06577f.
- Kalam, S.; Kamal, M. S.; Patil, S.; Hussain, S. M. Role of Counterions and Nature of Spacer on Foaming Properties of Novel Polyoxyethylene Cationic Gemini Surfactants. Processes 2019, 7 (8), 502.
- Kamal, M. S.; Hussain, S. M. S.; Fogang, L. T.; Malik, I. A. Development of Polyoxyethylene Zwitterionic Surfactants for High-Salinity High-Temperature Reservoirs. J. Surfactants Deterg. 2019, 22 (4), 795–806. https://doi.org/10.1002/jsde.12278.
- Hussain, S. M. S.; Kamal, M. S.; Fogang, L. T. Synthesis and Physicochemical Investigation of Betaine Type Polyoxyethylene Zwitterionic Surfactants Containing Different Ionic Headgroups. J. Mol. Struct. 2019, 1178, 83–88. https://doi.org/10.1016/j.molstruc.2018.09.094.
